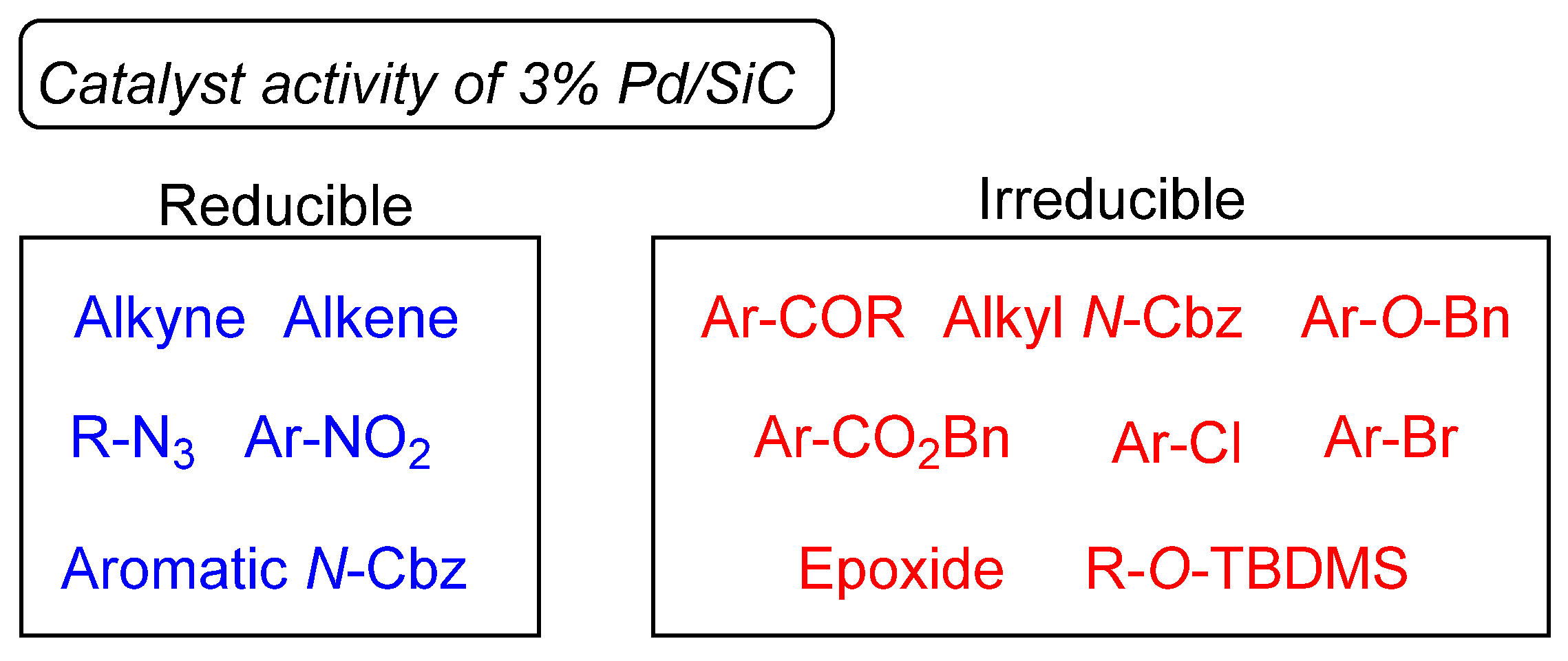
Catalysts | Free Full-Text | Development of Silicon Carbide-Supported Palladium Catalysts and Their Application as Semihydrogenation Catalysts for Alkynes under Batch- and Continuous-Flow Conditions

Syntheses of N^O-donor palladium(II) complexes and applications as recyclable catalysts in biphasic methoxycarbonylation of alkenes - ScienceDirect
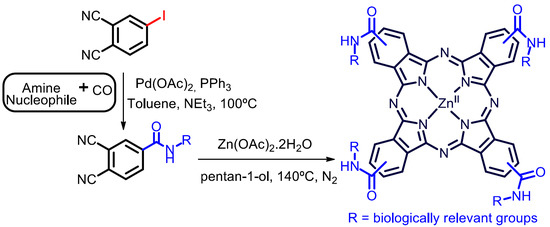
Catalysts | Free Full-Text | A New Tool in the Quest for Biocompatible Phthalocyanines: Palladium Catalyzed Aminocarbonylation for Amide Substituted Phthalonitriles and Illustrative Phthalocyanines Thereof

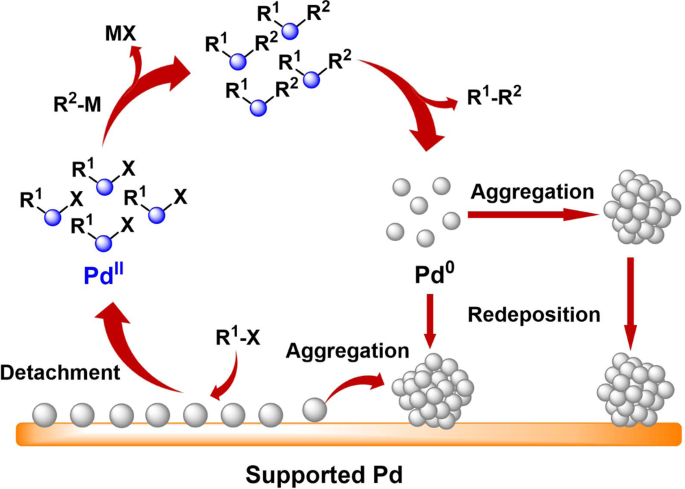
Can Palladium Acetate Lose Its “Saltiness”? Catalytic Activities of the Impurities in Palladium Acetate | Organic Letters

Can Palladium Acetate Lose Its “Saltiness”? Catalytic Activities of the Impurities in Palladium Acetate | Organic Letters

Can Palladium Acetate Lose Its “Saltiness”? Catalytic Activities of the Impurities in Palladium Acetate | Organic Letters

Synthesis and Characterization of Palladium(II) and Platinum(II) Adducts and Cyclometalated Complexes of 6,6′-Dimethoxy-2,2′-bipyridine: C(sp3)–H and C(sp2)–H Bond Activations | Organometallics

Linear α-Olefins Obtained with Palladium(II) Complexes Bearing a Partially Oxidized Tetraphosphane | Organometallics
Enhancing stability by trapping palladium inside N-heterocyclic carbene-functionalized hypercrosslinked polymers for heterogeneous C-C bond formations | Nature Communications

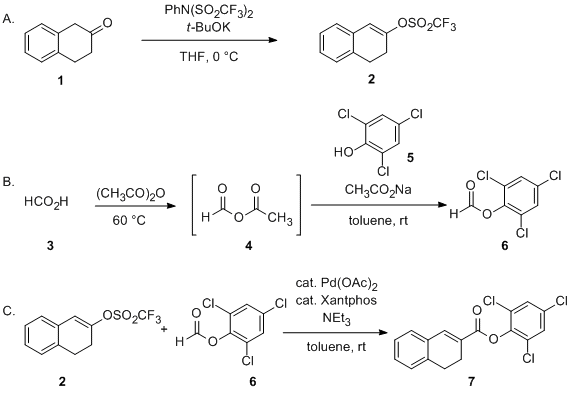
Palladium‐Catalyzed Asymmetric Hydrogenolysis of Aryl Triflates for Construction of Axially Chiral Biaryls - Li - 2023 - Angewandte Chemie International Edition - Wiley Online Library

Palladium Acetate Trimer: Understanding Its Ligand-Induced Dissociation Thermochemistry Using Isothermal Titration Calorimetry, X-ray Absorption Fine Structure, and 31P Nuclear Magnetic Resonance | Organometallics

Palladium/P,O-Ligand-Catalyzed Suzuki Cross-Coupling Reactions of Arylboronic Acids and Aryl Chlorides. Isolation and Structural Characterization of (P,O)-Pd(dba) Complex | The Journal of Organic Chemistry
Solution Processable Material Derived from Aromatic Triazole, Azomethine and Tris: Preparation and Hole-Buffering Application in Polymer Light-Emitting Diodes

Asymmetric Synthesis of Anti‐tuberculosis‐specific Drug TBAJ‐876 through Synergistic Li/Li Catalysis - Li - 2023 - Chinese Journal of Chemistry - Wiley Online Library